-
Question 1/50
5 / -1
lodine molecules are held in the crystals lattice by .......
-
Question 2/50
5 / -1
Which of the following statements about amorphous solid is incorrect
-
Question 3/50
5 / -1
A compound is made up of three elements X, Y and Z. X atoms are present at each corner of the unit cell, Y atoms are present on each edge and Z atoms are present in the centre of each face. The formula of the compound is:
-
Question 4/50
5 / -1
At 100℃, copper (Cu) has FCC unit cell structure with cell edge length of x Å. What is the approximate density of Cu (in g cm-3) at this temperature?
[Atomic Mass of Cu = 63.55 u]
-
Question 5/50
5 / -1
5 mL of N "HCl", 20 mL of N/2 "H2SO4" and 30 mL of N/3 "HNO3" are mixed together and volume made to one litre. The normality of the resulting solution is:
-
Question 6/50
5 / -1
Which of the following compounds has the highest value of Van’t Hoff factor?
-
Question 7/50
5 / -1
An aqueous solution of glucose is 10% in strength. The volume in which 1 g mole of it is dissolved will be
-
Question 8/50
5 / -1
Choose the incorrect statement from the following
-
Question 9/50
5 / -1
Change required to liberate 11.5 g sodium is
-
Question 10/50
5 / -1
Which is the correct Nernst equation for reaction taking place in the following cell
Mg(s) | Mg2+ (aq) || CI- (aq) | CI2(g) (1 atm)/Pt
-
Question 11/50
5 / -1
Which of the statements about solutions of electrolytes is not correct
-
Question 12/50
5 / -1
Which one of the following statement for order of reaction not correct
-
Question 13/50
5 / -1
A first order reaction has a specific reaction rate of 10-2 sec-1. How much time will it take for 20g of the reaction to reduce to 5g.
-
Question 14/50
5 / -1
In the given graph the activation energy, Ea for the reverse reaction will be

-
Question 15/50
5 / -1
Pick the appropriate choice about collision theory of reaction rates
-
Question 16/50
5 / -1
When excess of electrolyte is added to a colloid it
-
Question 17/50
5 / -1
The coagulation values in millimoles per litre of the electrolytes used for the coagulation of As2S3 are given below
I. (NaCl) = 52
II. (BaCl2) = 0.69
III, (MgSO4) = 0.22
The correct order of their coagulating power is:
-
Question 18/50
5 / -1
In Frevndlich Adsorption isotherm, the value of 1/n is
-
Question 19/50
5 / -1
Identify the incorrect statement among the following
-
Question 20/50
5 / -1
HI cannot be prepared by the action of conc. H2SO4 on KI because
-
Question 21/50
5 / -1
Which of following is used in the preparation of chlorine
-
Question 22/50
5 / -1
Ammonia forms the complex ion [(Cu(NH3)4]2+ with copper ions in alkaline solution but not in acidic solution.
-
Question 23/50
5 / -1
When EDTA solution is added to Mg2+ ion solution, then which of the following statements is not true
-
Question 24/50
5 / -1
The CFSE for octahedral [CoCl6]4– is 18,000 cm–1. The CFSE for tetrahedral [CoCl4]2– will be (x) ×102 cm–1 the value of x is:
-
Question 25/50
5 / -1
When 0.1 mol CoCl3(NH3)5 is treated with excess of AgNO3, 0.2 mol of AgCI are obtained. The conductivity of solution will correspond to
-
Question 26/50
5 / -1
The structural formula of hypophosphorous acid is
-
Question 27/50
5 / -1
Nitric acid can be obtained from ammonia via the formations of the intermediate compounds
-
Question 28/50
5 / -1
Identify the incorrect statement with respect to ozone
-
Question 29/50
5 / -1
Hot conc. H2SO4 acts as a moderately strong oxidizing agent. It oxidizes both metals and non-metals. Which of the following element is oxidized by conc. H2SO4 into two gaseous products:
-
Question 30/50
5 / -1
Which will undergo SN2 reaction faster
-
Question 31/50
5 / -1
Replacement of CI of chlorobenzene to give phenol requires drastic conditions but chlorine of 2, 4-dinitrochlorobenzene is readily replaced because
-
Question 32/50
5 / -1
What will be the product in the following reaction

-
Question 33/50
5 / -1
Acetyl bromide reacts with excess of CH3Mgl followed by treatment with a saturated solution of NH4Cl gives
-
Question 34/50
5 / -1
CH3CH2OH can be converted into CH3CHO by
-
Question 35/50
5 / -1
Phenol and NH3 reacts in presence of ZnCl2 at 300° C to produce
-
Question 36/50
5 / -1
When phenol is treated with CHCl3 and NaOH followed by acidification, salicylaldehyde is obtained. Which of the following species is involved in the above mentioned reaction as intermediates?
-
Question 37/50
5 / -1
An ether is more volatile than an alcohol having the same molecular formula. This is due to
-
Question 38/50
5 / -1
In the following reaction, final product is

-
Question 39/50
5 / -1
The reagent with which both acetaldehyde and acetone react easily is
-
Question 40/50
5 / -1
Which of the following statements is not true about Cannizzaro reaction?
-
Question 41/50
5 / -1
CH2 = CH(CH2)2 COOH and HBr reacts in presence of peroxide to give
-
Question 42/50
5 / -1
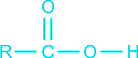
Select the correct statement about a carboxylic acid
-
Question 43/50
5 / -1
Acid anhydrides on reaction with primary amines give ______
-
Question 44/50
5 / -1
C3H9N cannot represent the following
-
Question 45/50
5 / -1
When ethyl amine is treated with methyl magnesium bromide, the product is:
-
Question 46/50
5 / -1
Which of the following reactions of glucose can be explained only by its cyclic structure
-
Question 47/50
5 / -1
Which enzyme can catalyse the conversion of glucose to ethanol?
-
Question 48/50
5 / -1
Which of the following statement is not correct
-
Question 49/50
5 / -1
Which of the following pair is/are correctly matched?
| Polymer | Uses of Polymer |
1. Terylene | Fabric |
2. Teflon | Non-stick cookware – plastics |
3. Melamine Formaldehyde Resin | Ceramic plastic material |
4. Nylon-6 | Synthetic rubber |
Select the correct code given below.
-
Question 50/50
5 / -1
Consider the following statements regarding Tranquilizers?
1. It relieves anxiety, stress, irritability or excitement by inducing a sense of well-being.
2. It is a neurologically inactive drug.
Which of the statements given above is/are not correct?



 Get latest Exam Updates
Get latest Exam Updates 





 ×
×






