WBJEE Question Paper
The West Bengal Joint Entrance Examination (WBJEE) is designed to assess your conceptual clarity, problem-solving ability, time management skills, etc., across various subjects. If you wish to pursue engineering, pharmacy, or architecture in affiliated colleges in this state, then you have to clear this examination. And for effective preparation, Mockers presents you with the WBJEE question paper in an online test format. It will help you to understand the structure, difficulty level, as well as question trends of the exam.
By attempting the WBJEE PYQs, you will also be able to get familiar with the exam pattern, marking scheme, negative marking rules, and so on. So, whether you are just starting your preparation or want to revise before the exam, practice with this resource can make a big difference in your overall performance.
Overview Of The West Bengal Joint Entrance Examination
In the table given below, you will find all the important information for this exam.
| ASPECT | DESCRIPTION |
| Examination Name | WBJEE: West Bengal Joint Entrance Examination |
| Examination Conducting Authority | WBJEEB: West Bengal Joint Entrance Examinations Board |
| Level of the Examination | State level |
| Examination Mode | Offline: OMR-based exam |
| Number of Papers | Two Paper 1: Mathematics Paper 2: Physics and Chemistry |
| Duration of the Examination | Total: 4 hours (2 hours for each paper) |
| Official Website | https://wbjeeb.nic.in/ |
WBJEE PYQs Available For Several Years
Here, at Mockers, you will get the opportunity to solve the WBJEE question paper with answer key from 2025 to 2012. The practice of these past papers will help you to understand the questions that are asked frequently and focus on the related topics more properly. With this, you can also analyze the level of difficulty across different years. By solving the papers on a regular basis, you will not only strengthen your clarity of the concepts but also improve your problem-solving speed and accuracy.
Pattern Of The Question Papers In WBJEE
All the questions in the West Bengal Joint Entrance Examination are multiple-choice questions. In each subject, there are three categories of questions. You can get more details about the pattern in the table below.
| Subject | Number of Questions | Total Number of Questions | Total Marks | ||
| Mathematics | 50 | 15 | 10 | 75 | 100 |
| Physics | 30 | 5 | 5 | 40 | 50 |
| Chemistry | 30 | 5 | 5 | 40 | 50 |
|
Note: In this exam, there are three types of categories in the questions for each subject. The marking scheme for all the categories is as follows:
|
|||||
WBJEE Question Paper With Answer Key
Our subject-matter experts provide the WBJEE question paper solved with answers. This means that after submitting the test, you can access all the correct answers along with detailed explanations. It will allow you to evaluate your performance in the test accurately. You just need to refer to these solutions, cross-check your responses, see your score, and understand where you went wrong. This process will help you in finding your weaker areas, correcting your conceptual mistakes, as well as avoiding similar errors in future test attempts.
WBJEE Eligibility And Academic Qualification Requirements
For your benefit, we have collected the information about eligibility as well as academic requirements for this examination.
ELIGIBILITY CRITERIA
- You must be an Indian or OCI citizen (subject to the Competent Authority's approval). Only unreserved seats in the All-India quota would be available to OCI candidates.
- You should have passed the 12th class (10+2) or an equivalent exam prior to that year, or must have taken the same in the particular year.
- The minimum age limit is seventeen years, and there is no upper age limit for this examination. However, if you want to take admission in a degree-level marine engineering course, then the upper age limit is 25 years.
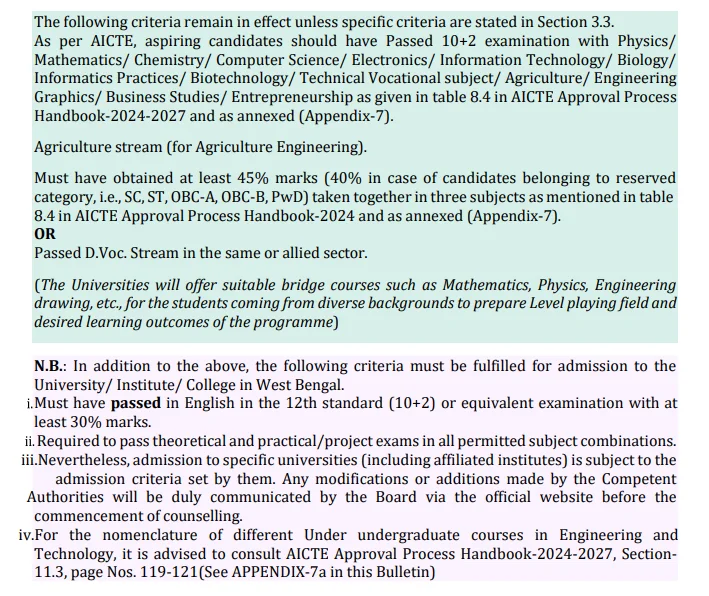
ACADEMIC CRITERIA
The images displayed below explain the criteria for admission into various engineering courses.

How To Attempt The WBJEE Past Year Question Paper On Mockers?
With the help of the steps mentioned below, you can take the test for the WBJEE past year question paper on our digital platform.
- You can reach Mockers by simply typing www.mockers.in on any of the web browsers.
- Once there, you should click on the ‘Navigation’ tab and select the ‘Exam Categories’ section.
- Now, from the various categories, you need to tap on the ‘Engineering’ one.
- This will further expand, and you can select the ‘WBJEE’ option.

- Then, a new web page will be available on your screen where you have to choose the ‘Previous Year Paper.’
- The WBJEE exam question paper from 2025-2012 can now be accessed by simply clicking on the ‘Explore’ icon.

- Finally, you have to tap on the ‘Start’ button of any WBJEE old question paper and attempt the test.

Important Topics In The WBJEE Question Paper Solved With Answers
Our experts have analyzed the online question papers with answers for WBJEE and mentioned their important topics across each subject in the table below.
| SUBJECT | IMPORTANT TOPICS |
| Mathematics | Algebra: A.P., G.P., H.P.: Definitions of A. P. and G.P.; General term; Summation of first n-terms of series; Arithmetic/Geometric series, A.M., G.M., and their relation; Infinite G.P. series and its sum. Logarithms: Definition; General properties; Change of base. Complex Numbers: Definition in terms of an ordered pair of real numbers and properties of complex numbers; Complex conjugate; Triangle inequality Polynomial equation: nth degree equation has exactly n roots (statement only) Quadratic Equations: Quadratic equations with real coefficients; Relations between roots and coefficients; Nature of roots; Formation of a quadratic equation Permutation and combination: Permutation of n different things taken r at a time (r ≤ n). The permutation of things is not all different. Principle of mathematical induction: Statement of the principle, proof by induction for the sum of squares, the sum of cubes of the first n natural numbers. Binomial theorem (positive integral index): Statement of the theorem, general term, middle term, equidistant terms, and properties of binomial coefficients. Matrices: Concepts of m x n (m ≤ 3, n ≤ 3) real matrices, operations of addition, scalar multiplication, and multiplication of matrices. Sets, Relations and Mappings: Idea of sets, subsets, power set, complement, union, intersection, and difference of sets, Venn diagram, De Morgan's Laws. Statistics and Probability: Measure of dispersion, mean, variance, and standard deviation, frequency distribution. Trigonometry: Trigonometric functions, addition and subtraction formulae, formulae involving multiple and submultiple angles. Coordinate geometry of two dimensions: Distance formula, section formula, area of a triangle, and condition of collinearity of three points in a plane. Polar coordinates, transformation from Cartesian to polar coordinates, and vice versa. Parallel transformation of axes. Coordinate geometry of three dimensions: Direction cosines and direction ratios, distance between two points, section formula, equation of a straight line, equation of a plane, and distance of a point from a plane. Differential calculus: Functions, domain and range, set of functions, composition of two functions, and inverse of a function. Integral calculus: Integration as a reverse process of differentiation, an indefinite integral of standard functions. Integration by parts. Integration by substitution and partial fractions. Differential Equations: Formation of ordinary differential equations, solution of homogeneous differential equations, separation of variables method, linear first-order differential equations. Application of Calculus: Tangents and normals, conditions of tangency. Determination of monotonicity, maxima, and minima. Differential coefficient as a measure of rate. Motion in a straight line with constant acceleration. Geometric interpretation of definite integral as area, calculation of area bounded by elementary curves and Straight lines. The area of the region included between two elementary curves. Vectors: Addition of vectors, scalar multiplication, dot and cross products, scalar triple product. |
| Physics | Physical World, Measurements, Units & dimensions: Physical World, Measurements, Units & Dimensions of physical quantities, dimensional analysis & its applications, error in measurements, significant figures. Kinematics: Scalars & vectors, representation of vectors in 3D, dot & cross product & their applications, elementary differential & integral calculus, time-velocity & relevant graphs, equations of motion with uniform acceleration. Laws of motion: Newton’s laws of motion, using algebra & calculus, inertial & non-inertial frames, conservation of linear momentum with applications, elastic & inelastic collisions, impulse centripetal force. The motion of the centre of mass, connected systems, Friction: Centre of mass of the two-particle system, motion of the connected system, torque, equilibrium of rigid bodies, moments of inertia of simple geometric bodies. Gravitation: Kepler’s laws, (only statement) universal law of gravitation, acceleration due to gravity (g), variation of g, gravitational potential & PE. Bulk properties of matter: Elasticity, Hooke’s law, Young’s modulus, bulk modulus, shear, rigidity modulus, Poisson’s ratio, elastic potential energy. Fluid pressure: Pressure due to a fluid column, buoyancy, Pascal’s law. Viscosity: Coefficient of viscosity, streamline & turbulent motion, Reynolds’ number, Stoke’s law, terminal velocity, Bernoulli’s theorem. Heat & Thermal Physics: Heat & temperature, thermal expansion of solids. Thermodynamics: Thermal equilibrium (Zeroth law of thermodynamics), heat, work & internal energy. 1st law of thermodynamics, isothermal & adiabatic processes, 2nd law of thermodynamics, reversible & irreversible processes. Kinetic theory of gases: Equation of state of a perfect gas, kinetic theory of gases, assumptions in Kinetic theory of gases, concept of pressure. & temperature; rms speed of gas molecules. Oscillations & Waves: Periodic motion – time period, frequency, time-displacement equation, Simple harmonic motion (S.H.M) & its equation; phase; SHM in different systems. Electrostatics: Conservation of electric charges, Coulomb's law force between two point charges, forces between multiple charges; superposition principle & continuous charge distribution. Current Electricity: Electric current, & conductor, drift velocity, mobility & their relation with electric current; Ohm's law, electrical resistance, Ohmic and non-Ohmic conductors, electrical energy & power. Magnetic effect of current: Concept of magnetic field, Oersted's experiment, Biot - Savart law & its application to current carrying circular loop; Ampere's law. Magnetics: Current loop as a magnetic dipole & its magnetic dipole moment, magnetic dipole moment of a revolving electron, magnetic field intensity due to a magnetic dipole bar magnet along its axis & perpendicular to its axis. Electromagnetic induction & alternating current: Electromagnetic induction; Faraday's laws, induced emf & current; Lenz's Law, eddy currents, self & mutual induction. Electromagnetic waves: Electromagnetic waves and their characteristics (qualitative ideas only), transverse nature of electromagnetic waves, electromagnetic spectrum, applications of the waves from the different parts of the spectrum. Optics I (Ray optics): Reflection of light, spherical mirrors, mirror formula. Refraction of light, total internal reflection & its applications, optical fibres, refraction at spherical surfaces, lenses, thin lens formula, lensmaker's formula. Newton's relation. Optics II (Wave Optics): Scattering of the light-blue colour of the sky, the elementary idea of the Raman effect; wave optics: wavefront & Huygens' principle. Atomic Physics: Alpha-particle scattering experiment, Rutherford's nuclear atom model of the atom; Bohr model of the hydrogen atom, energy levels in a hydrogen atom, hydrogen spectrum, continuous & characteristic x-rays. Nuclear Physics: Composition & size of nucleus, atomic masses, isotopes, isobars, isotones, radioactivity - alpha, beta & gamma particles/ rays & their properties; radioactive decay law; mass-energy relation, mass defect. Solid state Electronics: Energy bands in solids (qualitative ideas only), conductors, insulators & semiconductors; semiconductor diode – I-V characteristics in forward & reverse bias, diode as a rectifier; I-V characteristics of LED, photodiode, solar cell & Zener diode; Zener diode as a voltage regulator, junction transistor (BJT). |
| Chemistry | Atoms, Molecules and Chemical Arithmetic: Dalton’s atomic theory; Gay Lussac’s law of gaseous volume; Avogadro’s Hypothesis and its applications. Atomic mass; Molecular mass; Equivalent weight; Valency. Atomic Structure: Concept of Nuclear Atom – electron, proton, and neutron (charge and mass), atomic number, Rutherford’s model and its limitations; Extra nuclear structure; Line spectra of hydrogen atom. Quantization of energy (Planck’s equation E = hν). Radioactivity and Nuclear Chemistry: Radioactivity α-, β-, γ rays and their properties; Artificial transmutation; Rate of radioactive decay, decay constant, half-life, and average life period of radio-elements; Units of radioactivity; Numerical problems. The Periodic Table and Chemical Families: Modern periodic law (based on atomic number); Modern periodic table based on electronic configurations, groups (Gr. 1-18) and periods. Types of elements – representative (s-block and p-block), transition (d-block) elements, and inner transition. Chemical Bonding and Molecular Structure: Valence electrons, the Octet rule, electrovalent, covalent, and coordinate covalent bonds with examples; Properties of electrovalent and covalent compounds. Limitations of the Octet rule (examples); Fajans Rule. Coordination Compounds: Introduction, Double salts and complex salts, coordination compounds (examples only), Werner's theory, coordination number (examples of coordination numbers 4 and 6 only). Solid State: Classification of solids based on different binding forces: molecular, ionic, covalent, and metallic solids, amorphous and crystalline solids (elementary idea). Liquid State: Vapour pressure, viscosity, and surface tension (qualitative idea only, no mathematical derivations). Gaseous State: Measurable properties of gases. Boyle’s Law and Charles Law, absolute scale of temperature, kinetic theory of gases, ideal gas equation – average, root mean square, and most probable velocities, and their relationship with temperature. Chemical Energetics: Conservation of energy principle, energy changes in physical and chemical transformations. The first law of thermodynamics: Internal energy, work, and heat, pressure – volume work; Enthalpy. Chemical Equilibria: The Law of mass action, dynamic nature of chemical equilibria. Equilibrium constants, Le Chatelier's Principle. Equilibrium constants of gaseous reactions. Chemical Dynamics: Factors affecting the rate of chemical reactions (concentration, pressure, temperature, catalyst), Concept of collision theory. Arrhenius equation and concept of activation energy. Order and molecularity (determination excluded); First-order reactions, rate constant, halflife (numerical problems), examples of first-order and second-order reactions. Physical Chemistry of Solutions: Colloidal Solutions – Differences from true solutions; Hydrophobic and hydrophilic colloids (examples and uses); Coagulation and peptization of colloids. Electrolytic Solutions – Specific conductance, equivalent conductance, ionic conductance, Kohlrausch’s law, Faraday’s laws of electrolysis, applications. Numerical problems. Non-electrolytic Solutions – Types of solution, vapour pressure of solutions. Raoult’s Law; Colligative properties. Ionic and Redox Equilibria: Ionic equilibria – ionization of weak electrolytes, Ostwald’s dilution law. Ionization constants of weak acids and bases, ionic product of water, and the pH scale. Redox Equilibria: Oxidation – Reduction reactions as electron transfer processes, oxidation numbers, balancing of redox reactions by oxidation number and ion-electron methods. Hydrogen: Position of hydrogen in the periodic table, occurrence, isotopes, preparation, properties, and uses of hydrogen, hydrides, ionic, covalent, and interstitial. Chemistry of Non-Metallic Elements and their Compounds: Carbon – occurrence, isotopes, allotropes (graphite, diamond, fullerene); CO and CO2 production, properties, and uses. Oxygen and Sulphur – Occurrence, isotopes, allotropic forms, isolation from natural sources and purification, properties, and reactions of the free elements. Halogens – a comparative study, occurrence, physical states, and chemical reactivities of the free elements, peculiarities of fluorine and iodine. Chemistry of Metals: General principles of metallurgy – occurrence, concentration of ores, production and purification of metals, mineral wealth of India. Principles of chemistry are involved in electroplating, anodizing, and galvanizing. Preparation and properties of K2Cr2O7 and KMnO4. Lanthanoids – Electronic configuration, oxidation states, chemical reactivity, and lanthanoid contraction and its consequences. Actinoids – Electronic configuration, oxidation states, and comparison with lanthanoids. Chemistry in Industry: Large-scale production (including physicochemical principles where applicable, omitting technical details) and uses of Sulphuric acid (contact process), Ammonia (Haber’s process), Nitric acid (Ostwald’s process), sodium bicarbonate and sodium carbonate (Solvey process). |
Benefits Of Solving The WBJEE Exam Question Paper
We offer you the free online question papers with answers for WBJEE as well as the WBJEE mock tests in a structured format so that you can maximize your learning as well as performance. You can take these tests anytime and from anywhere, which will make your preparation flexible and consistent. Here are some more benefits of solving these papers on our platform.
- We present you with the WBJEE question paper after collecting it from the official sources. This means you get to practice high-quality questions that reflect the real levels of difficulty. By solving the papers, you can make sure that your preparation remains relevant as well as exam-oriented.
- On Mockers, practice of the WBJEE PYQs will help you learn how to allocate time in an efficient manner across the subjects. With the built-in timer, you will be able to maintain your speed without compromising on accuracy. This is an important skill to crack the West Bengal Joint Entrance Examination.
- We provide you with the result of your attempted WBJEE question paper with answer key as soon as you submit the test. This way, you can understand your score as well as accuracy quickly. The instant feedback also encourages you to practice on a regular basis and adjust your preparation strategy.
- You get the WBJEE question paper solved with answers on our website. These clear and well-explained solutions help you to understand not just the correct answer but also the logic behind it. With this, you will be able to solidify your clarity of the concepts as well as prevent the same mistakes.
- Once you analyze your WBJEE past year question paper performance, you can notice the topics where you excel and areas that require more revision quite easily. Through this targeted approach, you can save a lot of time and make your preparation more efficient as compared to random practice.
Different Stages To Solve The WBJEE Old Question Paper
During your preparation journey, practicing with the WBJEE exam question paper is not just about how many papers you attempt, but also when you attempt them. Your purpose as well as approach to solving the papers need to change as you progress from learning concepts to mastering exam strategy. We have mentioned some of the important stages where you should solve the past papers.
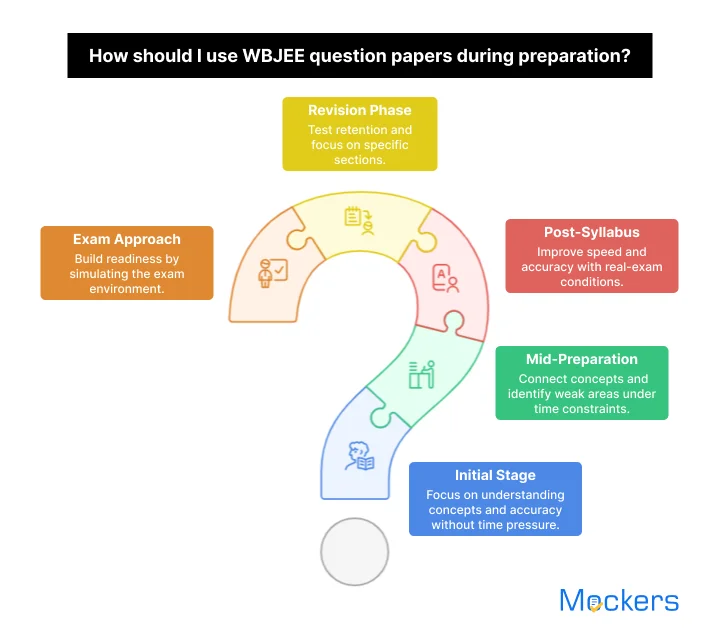
- When you are in the initial stages of your preparation, you should practice with the WBJEE old question paper in a topic-wise manner. You should also not worry about the time pressure at this time. During this phase, your goal is to understand the application of concepts. You need to focus on accuracy, logical thinking, learning new approaches to solve problems, and so on.
- Once you have covered the major portion of the syllabus, you need to start solving the WBJEE past year papers online test as per the time. You should learn how to connect concepts from different chapters and find the patterns that repeat. While practicing the papers in this phase, you will be able to highlight your weaker areas that need revision and solidify the interlinked topics.
- You should attempt the online question papers with answers for WBJEE fully after completing the entire syllabus. In this phase, you should apply strict time limits as well as follow the structure of the real examination. Your focus should now shift to improving speed, accuracy, question selection strategy, etc. Always keep in mind that this is the stage where you have to do performance analysis after every test.
- During the revision phase, solving the free online question papers with answers for WBJEE will help you test your retention as well as conceptual clarity. You may focus on solving selected sections or past wrong questions rather than attempting the full papers. Through this targeted practice, you can make sure to revise in an efficient manner as well as avoid wasting time.
- As the exam approaches, you must solve the WBJEE question paper in a real-exam environment. This means you should attempt the papers at the same time of day as the actual exam so that you can build your mental as well as physical readiness. In this stage, you will be able to refine your time management, reduce your exam anxiety, boost your confidence, and so on.
Centers Where The WBJEE Is Conducted
All around the state of West Bengal, there are various centers for this examination. You can have a look at the table below for more details.
| DISTRICTS | ZONES |
| Alipurduar | Alipurduar |
| Bankura | Bankura, Bishnupur |
| Birbhum | Bolpur, Suri |
| Cooch Behar | Cooch Behar |
| Dakshin Dinajpur | Balurghat |
| Darjeeling | Kurseong, Siliguri |
| Hooghly | Arambagh, Bandel/Chinsurah, Serampore |
| Howrah | Howrah Maidan/Shibpur, Salkia/Bally/Uttarpara, Santragachi/Domjur, Uluberia |
| Jalpaiguri | Jalpaiguri |
| Jhargram | Jhargram |
| Kalimpong | Kalimpong |
| Kolkata | Central Kolkata: Moulali/Beliaghata/Narkel/Danga/Phool Bagan/Kakurgachi/Park Circus North Kolkata: Shyambazaar/Bagh Bazar/Girish Park/Burra Bazar/College Street/Sealdah Salt Lake/New Town: Salt Lake/Lake Town/New Town/Rajar Hat West Kolkata: Joka/Behala/Alipore/Chetla/Khidirpore/Budge Budge South Kolkata: Ballygaunge/Minto Park/Bhowanipore/Tollygaunge/Jadavpur |
| Malda | Malda |
| Murshidabad | Berhampur, Jiaganj, Raghunathganj |
| Nadia | Kalyani, Krishnagar, Nabadwip |
| North 24 Parganas | Ashoknagar, Barasat (Airport/Madhyamgram/Barasat), Barrackpur (Dum Dum Jn. to Barrackpur), Basirhat |
| Paschim Burdwan | Asansol, Durgapur |
| Paschim Medinipur | Garbeta, Kharagpur, Medinipur |
| Purba Burdwan | Burdwan |
| Purba Medinipur | Contai, Haldia, Tamluk |
| Purulia | Purulia |
| South 24 Parganas | Garia/Sonarpur/Baruipur, Jainagar |
| Uttar Dinajpur | Raiganj |
| Other States | Assam: Silchar Tripura: Agartala |
Note:
|
|
| Howrah | Salkia/Bally/Uttarpura |
| Kolkata | Salt Lake/New Town: Salt Lake/Lake Town/New Town/Rajar Hat South Kolkata: Ballygaunge/Minto Park/Bhowanipore/Tollygaunge/Jadavpur West Kolkata: Joka/Behala/Alipore/Chetla/Khidirpore/Budge Budge |
| Paschim Burdwan | Asansol, Durgapur |
| Paschim Medinipur | Kharagpur |
Conclusion
Attempts at the WBJEE PYQs on our digital platform will give you a clear understanding of the exam pattern, marking scheme, question difficulty, topic-wise weightage, etc. With this, you will be able to align your preparation with the requirements of the actual exam. Moreover, analysis of these papers will enable you to recognize the concepts that are frequently asked, which allows you to focus on the high-priority areas. By combining the papers with a proper study plan as well as regular revision, you can boost your performance in the exam significantly.
 Bank
Bank







 SSC
SSC









 Railway
Railway







 State
State




 Other
Other


 Teaching
Teaching




 Insurance
Insurance


 Medical
Medical



 Engineering
Engineering



















 Defence
Defence





 GATE
GATE





 NTA CUET
NTA CUET

 UPSC
UPSC

 MBA Entrance
MBA Entrance

















 LAW
LAW

 DSSSB PRT 2025
DSSSB PRT 2025
 RRB JE 2025
RRB JE 2025
 RRB Technician CBT-I 2025
RRB Technician CBT-I 2025
 UP Police SI (दरोगा) 2025
UP Police SI (दरोगा) 2025
 SSC GD 2025-26
SSC GD 2025-26
 RRB NTPC 2026
RRB NTPC 2026
 CDS I 2026
CDS I 2026
 NDA I 2026
NDA I 2026
 SSC CGL Tier-I 2026
SSC CGL Tier-I 2026
 SSC JE 2026
SSC JE 2026













 Get latest Exam Updates
Get latest Exam Updates 



